Anodising Aluminium.
Anodising is a process that can be carried out by the average home constructor quite cheaply, and as the chemicals can be used again and again, the cost is even cheaper than painting. There is also the advantage that one can carry out work on the item as soon as the process is finished and not have to wait for the paint to dry.
The only equipment required are rubber gloves, goggles, a piece of lead sheet, and some plastic containers for the chemicals and for clean water.
Chemicals:
The chemicals used are highly corrosive, so the dangers involved in their use cannot be stressed more vehemently, so great care must be taken at all times and all operations carried out in a well ventilated area. In the case of accidental contact, the chemical must be washed off immediately especially if splashed into the eyes, and a visit to your A & E department is advisable,
The author used plastic buckets for all processing and put the used chemicals back into their bottles as soon as he had completed each stage of the process. The first and most important rule to observe apart from safety is absolute cleanliness, or the final product will be smeary and the colour uneven.
Preparation:
First thoroughly clean the article by brushing it all over with steel wool so that there is an even matt finish all over the surfaces to be treated. Care taken at this stage will be repaid in the appearance of the finished article.
The cleaned article is next placed in a strong solution of caustic soda, this is not a critical operation, but the solution must be strong enough to etch the aluminium. Leave the article in the solution for about ¼ of an hour, remove and thoroughly rinse it and place on one side. It is not advisable to touch it with bare hands at this stage as finger marks will spoil the finish.
A preparation of dilute sulphuric acid diluted in the following proportions of one part sulphuric acid to seven parts by volume distilled water.
N.B. Always add the acid to the water, not the reverse as the liquid will become very hot and may erupt violently. Place the dilute acid to one side to cool if necessary.
Also prepare a solution of copper sulphate in the proportions of 2% copper sulphate to 98% of water by weight, place safely on one side for use later.
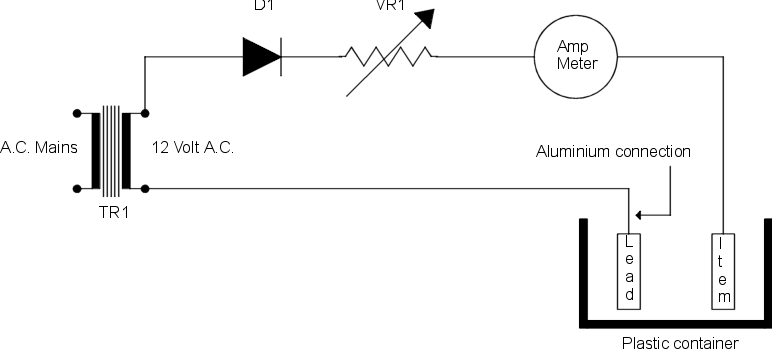
A 12 Volt D.C. supply capable of providing about 8 Amps is required in order to form the oxide layer by electrolysis. This will accept the dye which will give the anodised effect.
If an 8 Amp D.C. supply is not available, you will need to extend the process time in order to take into account the lower current. Please note that the oxide layer when formed does not readily conduct electricity, so a convenient position on the article should be chosen where it can be securely connected to the supply without spoiling the overall appearance.
Process:
With the dilute acid placed in one of the buckets, immerse the article and connect it to the positive supply with a spare piece of aluminium. The reason that aluminium is specified is that a connection made with any other metal would cause a blemish.
A small piece of lead is used for the negative connection and the current is adjusted to between 6 and 7 Amps. This current is passed for about 15 minutes or longer if only a lower current is available.
Dyeing:
The next stage is the dyeing of the article in the colour of choice. The dye recommended is a readily available domestic fabric colourant, although it may be possible to use inkjet printer refill ink, this has not been tried but may be worth the experiment if it's available.
Dissolve the fabric dye in about ½ gallon of water which has just come off the boil to which a desert spoonful of acetic acid (vinegar) has been added. This assists in the fixing of the dye and helps to render it stable in direct sunlight.
Place the article in this solution for about ½ hour, then after washing it thoroughly place it in the copper sulphate solution for about 10 minutes.
These chemicals can be used many times until they become discoloured and the dye loses it's strength. The article may be drilled but it is recommended that any bending is carried out before anodising.
Disposal:
Please contact your local authority for instructions as to how to safely dispose of the chemicals when they are no longer required.